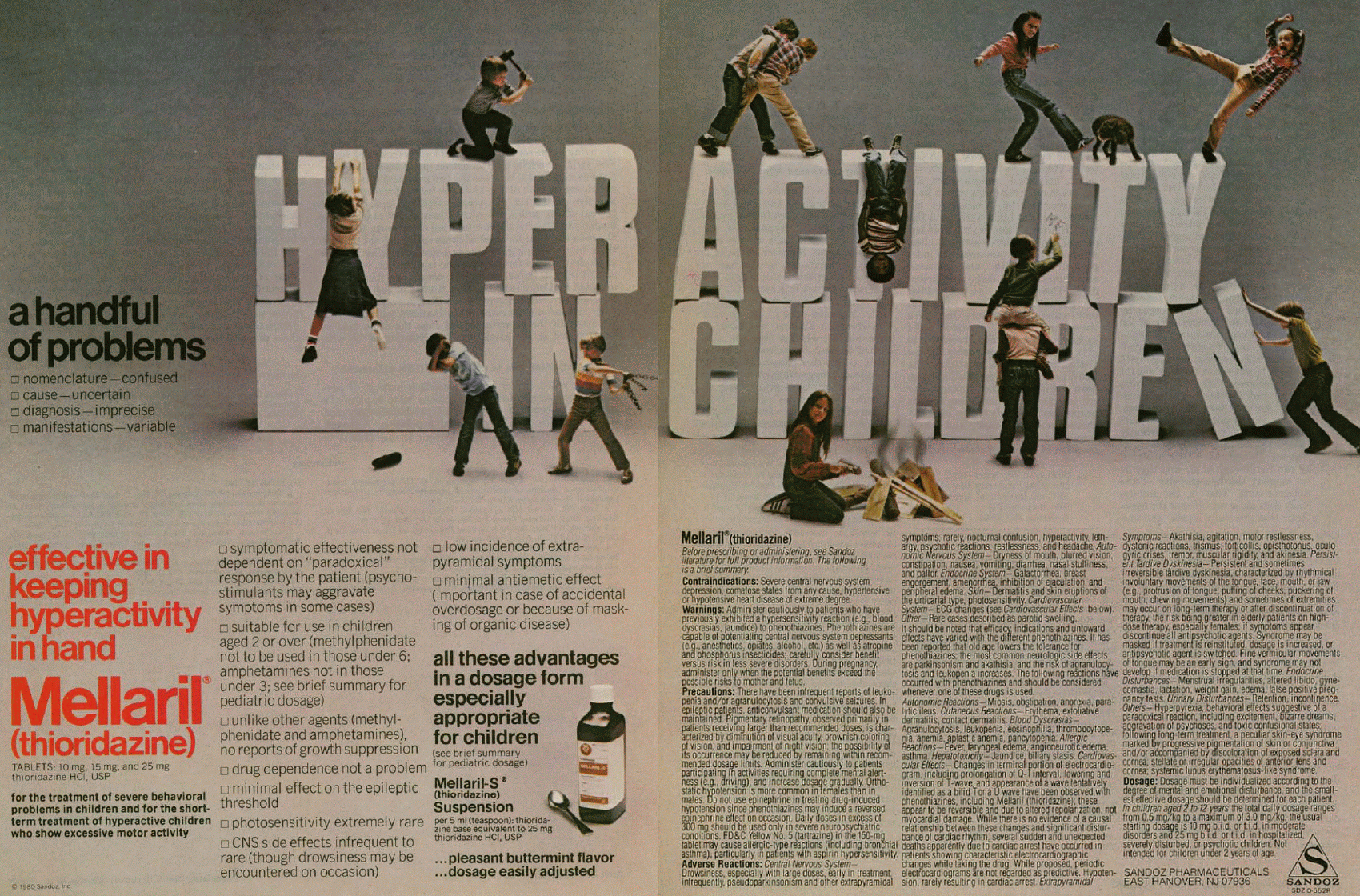
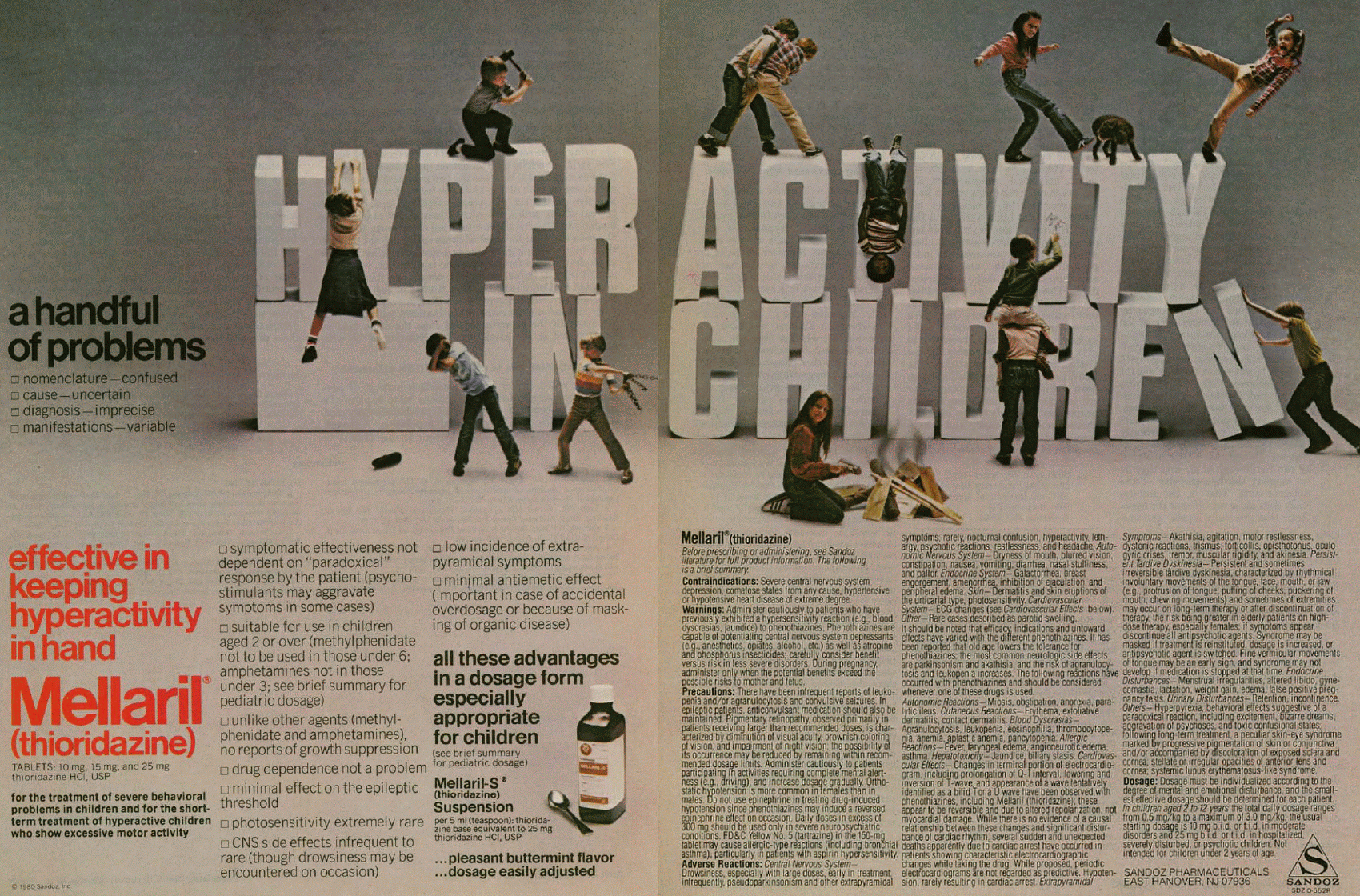
|
|
Mellaril advertisement, 1980. Archives of General Psychiatry, Vol. 37, No. 10, pp. 1198-1199. Hyperactivity in children ~ a handful of problems * nomenclature -- confused * cause -- uncertain * diagnosis -- imprecise * manifestations -- variable effective in keeping hyperactivity in hand Mellaril® (thioridazine) for the treatment of severe behavioral problems in children and for the short-term treatment of hyperactive children who show excessive motor activity * symptomatic effectiveness not dependent on "paradoxical" response by the patient (psycho-stimulants may aggravate symptoms in some cases) *suitable for use in children aged 2 or over (methylphenidate not to be used in those under 6; amphetamines not in those under 3; see brief summary for pediatric dosage) * unlike other agents (methylphenidate and amphetamines), no reports of growth suppression * minimal effect on the epileptic threshold *photosensitivity extremely rare * CNS side effects infrequent to rare (though drowsiness may be encountered on occasion) * low incidence of extrapyramidal symptoms * minimal antiemetic effect (important in case of accidental overdosage or because of masking of organic disease ) all these advantages in a dosage form especially appropriate for children (see brief summary for pediatric dosage) ...pleasant buttermint flavor ...dosage easily adjusted Adverse Reactions: Central Nervous System -- Drowsiness, especially with large doses, early in treatment. Infrequently, pseudoparkinsonism and other extrapyramidal symptoms; rarely, nocturnal confusion, hyperactivity, lethargy, psychotic reactions, restlessness, and headache. Autonomic Nervous System -- Dryness of mouth, blurred vision, constipation, nausea, vomiting, diarrhea, nasal stuffiness, and pallor. Endocrine system -- Galactorrhea, breast engorgement, amenorrhea, inhibition of ejaculation, and peripheral edema. Skin -- Dermatitis and skin eruptions of the urticarial type, photosensitivity. Cardiovascular System -- ECG changes (see Cardiovascular Effects below). Other -- Rare cases described as parotid swelling. It should be noted that efficacy indications and untoward effect have varied with the different phenothiazines. It has been reported that old age lowers the tolerance for phenothiazines; the most common neurologic side effects are parkinsonism and akathisia, and the risk of agranulocytosis and leukopenia increases. The following reactions have occurred with phenothiazines and should be considered whenever one of these drugs is used. Autonomic Reactions -- Miosis, obstipation, anorexia, paralytic ileus. Cutaneous Reactions -- Erythema, exfoliative dermatitis, contact dermatitis. Blood Dyscrasias -- Agranulocytosis, leukopenia, eosinophilia, thrombocytopenia, anemia, aplastic anemia, pancytopenia. Allergic Reactions -- Fever, laryngeal edema, angioneurotic edema, asthma. Hepatoxicity -- Jaundice, biliary stasis. Cardiovascular Effects -- Changes in terminal portion of electrocardiogram, including prolongation of Q-T interval, lowering and inversion of T-wave, and appearance of a wave tentatively identified as a bifid T or a U wave have been observed with phenothiazines, including Mellaril (thioridazine); these appear to be reversible and due to altered repolarization, not myocardial damage. While there is no evidence of a causal relationship between these changes and significant disturbance of cardiac rhythm, several sudden and unexpected deaths apparently due to cardiac arrest have occurred in patients showing characteristic electrocardiographic changes while taking the drug. While proposed, periodic electrocardiograms are not regarded as predictive. Hypotension, rarely resulting in cardiac arrest. Extrapyramidal Symptoms -- Akathisia, agitation, motor restlessness, dystonic reactions, trismus, torticollis, opisthotonus, oculogyric crises, tremor, muscular rigidity, and akinesia. Persistent Tardive Dyskinesia -- Persistent and sometimes irreversible tardive dyskinesia, characterized by rhythmical involuntary movements of the tongue, face, mouth, or jaw (e.g., protrusion of tongue, puffing of cheeks, puckering of mouth, chewing movements) and sometimes of extremities may occur on long-term therapy or after discontinuation of therapy, the risk being greater in elderly patients on high-dose therapy, especially females; if symptoms appear, discontinue all antipsychotic agents. Syndrome may be masked if treatment is reinstituted, dosage in increased, or antipsychotic agent is switched. Fine vermicular movements of tongue may be an early sign, and syndrome may not develop if medication is stopped at that time. Endocrine Disturbances Menstrual irregularities, altered libido, gynecomastia, lactation, weight gain, edema, false positive pregnancy tests. Urinary Disturbance -- Retention, incontinence. Others -- Hyperpyrexia; behavioral effects suggestive of a paradoxical reaction, including excitement, bizarre dreams, aggravation of psychoses, and toxic confusional states; following long-term treatment, a peculiar skin-eye syndrome marked by progressive pigmentation of skin or conjunctiva and/or accompanied by discoloration of exposed sclera and cornea; stellate or irregular opacities of anterior lens and cornea; systemic lupus erythematosus-like syndrome. Dosage: Dosage must be individualized according to the degree of mental and emotional disturbance, and the smallest effective dosage should be determined for each patient. In children aged 2 to 12 years the total daily dosage ranges from 0.5 mg/kg to a maximum of 3.0 mg/kg; the usual starting dosage is 10 mg b.i.d. or t.i.d. in moderate disorders and 25 mg b.i.d. or t.i.d. in hospitalized, severely disturbed, or psychotic children. Not intended for children under 2 years of age. Sandoz Pharmaceuticals East Hanover, NJ 07936 |