
|
|
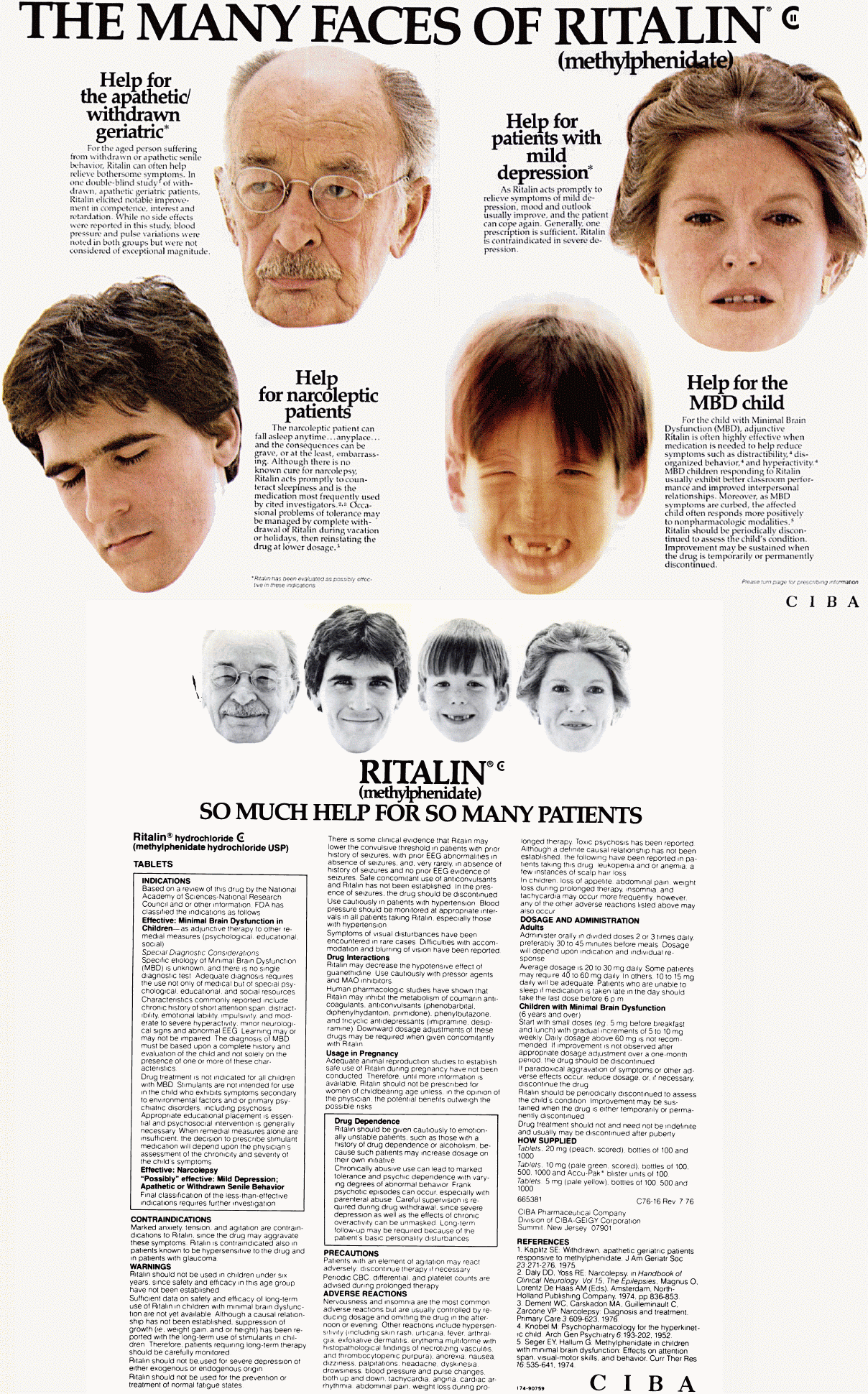
Ritalin advertisement, 1979. Psychosomatics, Vol. 20, No. 3. The many faces of RITALIN® (methylphenidate) Help for the apathetic/withdrawn geriatric* For the aged person suffering from withdrawn or apathetic senile behavior, Ritalin can often help relieve bothersome symptoms. In one double-blind study of withdrawn, apathetic geriatric patients, Ritalin elicited notable improvement in competence, interest and retardation. While no side effects were reported in this study, blood pressure and pulses variations were noted in both groups but were not considered of exceptional magnitude. Help for patients with mild depression* As Ritalin acts promptly to relieve symptoms of mild depression, mood and outlook usually improve, and the patient can cope again. Generally, one prescription is sufficient. Ritalin is contraindicated in severe depression. Help for narcoleptic patients The narcoleptic patient can fall asleep anytime... anyplace... and the consequences can be grave, or at the least, embarrassing. Although there is no known cure for narcolepsy, Ritalin acts promptly to counteract sleepiness and is the medication most frequently used by cited nvestigators.(2,3) Occasional problems of tolerance may be managed by complete withdrawal of Ritalin during vacation or holidays, then reinstating the drug at lower dosage. Help for the MBD child For the child with Minimal Brain Dysfnction (MBD), adjunctive Ritalin is often highly effective when medication is needed to help reduce symptoms such as distractibility,(4) disorganized behavior,(4) and hyperactivity.(4) MBD children responding to Ritalin usually exhibit better classroom performance and improved interpersonal relationships. Moreover, as MBD symptoms are curbed, the affected child often responds more positively to nonpharmacologic modalities.(5) Ritalin should be periodically discontinued to assess the child's condition. Improvement may be sustained when the drug is temporarily or permanently discontinued. *Ritalin has been evaluated as possibly effective in these indications. Please turn page for prescribing information. RITALIN® (methylphenidate) ~ so much help for so many patients Ritalin® hydrochloride (methylphenidate hydrochloride USP) INDICATIONS ~ Based on a review of this drug by the National Academy of Science-National Research Council and or other information, FDA has classified the indications as follows Effective: Minimal Brian Dysfunction in Children -- as adjunctive therapy to other remedial measures (psychological, educational, social) Special Diagnostic Considerations Specific etiology of Minimal Brain Dysfunction (MBD) is unknown, and there is no single diagnostic test. Adequate diagnosis requires the use not only of medical but of special psychological, educational, and social resources. Characteristics commonly reported include chronic history of short attention span, distractibility, emotional lability, impulsivity, and moderate to severe hyperactivity, minor neurological signs and abnormal EEG. Learning may or may not be impaired. The diagnosis of MBD must be based upon a complete history and evaluation of the child and not solely on the presence of one or more of these characteristics. Drug treatment is not indicated for all children with MBD. Stimulants are not intended for use in the child who exhibits symptoms secondary to environmental factors and/or primary psychiatric disorders including psychosis. Appropriate educational placement is essential and psychosocial intervention is generally necessary. When remedial measures alone are insufficient, the decision to prescribe stimulant medication will depend upon the physician's assessment of the chronicity and severity of the child's symptoms. Effective: Narcolepsy "Possibly" effective: Mild Depression; Apathetic or Withdrawn Senile Behavior Final classification of the less-than-effective indications requires further investigation. CONTRAINDICATIONS ~ Marked anxiety, tension, and agitation are contraindications to Ritalin, since the drug may aggravate these symptoms. Ritalin is contraindicated also in patients known to be hypersensitive to the drug and in patients with glaucoma. WARNINGS ~ Ritalin should not be used in children under six years, since safety and efficacy in this age group have not been established. Sufficient data on safety and efficacy of long-term use of Ritalin in children with minimal brain dysfunction are not yet available. Although a causal relationship has not been established, suppression of growth (i.e., weight gain, and/or height) has been reported with the long-term use of stimulants in children. Therefore, patients requiring long-term therapy should be carefully monitored. Ritalin should not be used for severe depression of either exogenous or endogenous origin. Ritalin should not be used for the prevention or treatment of normal fatigue states. There is some clinical evidence that Ritalin may lower the convulsive threshold in patients with prior history of seizures, with prior EEG abnormalities in absence of seizures, and, very rarely, in absence of history of seizures and no prior EEG evidence of seizures. Safe concomitant use of anticonvulsants and Ritalin has not been established. In the presence of seizures, the drug should be discontinued. Use cautiously in patients with hypertension. Blood pressure should be monitored at appropriate intervals in all patients taking Ritalin, especially those with hypertension. Symptoms of visual disturbances have been encountered in rare cases. Difficulties with accommodation and blurring of vision have been reported. Usage in Pregnancy Adequate animal reproduction studies to establish safe use of Ritalin during pregnancy have not been conducted. Therefore, until more information is available, Ritalin should not be prescribed for women of childbearing age unless, in the opinion of the physician, the potential benefits outweigh the possible risks. Drug Dependence: Ritalin should be given cautiously to emotionally unstable patients, such as those with a history of drug dependence or alcoholism, because such patients may increase dosage on their own initiative. Chronically abusive use can lead to marked tolerance and physic dependence with varying degrees of abnormal behavior. Frank psychotic episodes can occur, especially with parenteral abuse. Careful supervision is required during drug withdrawal, since severe depression as well as the effects of chronic over-activity can be unmasked. Long-term follow-up may be required because of the patient's basic personality disturbances. PRECAUTIONS ~ Patients with an element of agitation may react adversely; discontinue therapy if necessary. Periodic CBC, differential, and platelet counts are advised during prolonged therapy. ADVERSE REACTIONS ~ Nervousness and insomnia are the most common adverse reactions but are usually controlled by reducing dosage and omitting the drug in the afternoon or evening. Other reactions include hypersensitivity (including skin rash, urticaria, fever, arthralgia, exfoliative dermatitis, erythema multiforme with histopathological findings of necrotizing vasculitis, and thrombocytopenic purpura); anorexia; nausea; dizziness; palpitations; headache; dyskinesia; drowsiness; blood pressure and pulse changes, both up and down; tachycardia; angina; cardiac arrhythmia; abdominal pain; weight loss during prolonged therapy. Toxic psychosis has been reported. Although a definite causal relationship has not been established, the following have been reported in patients taking this drug: leukopenia and/or anemia; a few instances of scalp hair loss. In children, loss of appetite, abdominal pain, weight loss during prolonged therapy, insomnia, and tachycardia may occur more frequently; however, any of the other adverse reactions listed above may also occur. DOSAGE AND ADMINISTRATION ~ Administer orally in divided doses 2 or 3 times daily, preferably 30 to 45 minutes before meals. Dosage will depend upon indication and individual response. Average dosage is 20 to 30 mg daily. Some patients may require 40 to 60 mg daily. In others, 10 to 15 mg daily will be adequate. Patients who are unable to sleep if medication is taken late in the day should take the last dose before 6 p.m. Children with Minimal Brain Dysfunction (6 years and over) Start with small doses (eg. 5 mg before breakfast and lunch) with gradual increments of 5 to 10 mg weekly. Daily dosage above 60 mg is not recommended. If improvement is not observed after appropriate dosage adjustment over a one-month period, the drug should be discontinued. If paradoxical aggravation of symptoms or other adverse effects occur, reduce dosage or, if necessary, discontinue the drug. Drug treatment should not and need not be indefinite and usually may be discontinued after puberty. HOW SUPPLIED ~ Tablets, 20 mg (peach, scored), bottles of 100 and 1000. Tablets, 10 mg (pale green, scored), bottles of 100. Tablets, 5mg (pale yellow), bottles of 100, 500 and 1000. CIBA Pharmaceutical Company ~ Division of CIBA-GEIGY Corporation ~ Summit, New Jersey 07901 References 1. Kaplitz SE: Withdrawn, apathetic geriatric patients responsive to methylphenidate. J Am Geriatr Soc 23:271-276. 1975. 2. Daly DD, Yoss RE: Narcolepsy. in Handbook of Clinical Neurology, Vol 15, The Epilepsies, Magnus O. Lorentz De Haas AM (Eds). Amsterdam, North-Holland Publishing Company, 1974, pp 836-853. 3. Dement WC, Carskadon MA, Guilleminault C, Zarcon VP: Narcolepsy: Diagnosis and treatment. Primary Care 3:609-623, 1976. 4. Knobel M: Psychopharmacology for the hyperkinetic child. Arch Gen Psychiatry 6:193-202, 1952. 5. Seger EY, Hallum G: Methylphenidate in children with minimal brain dysfunction: Effects on attention span, visual-motor skills, and behavior. Curr Ther Res 16:535-641, 1074. |