
|
|
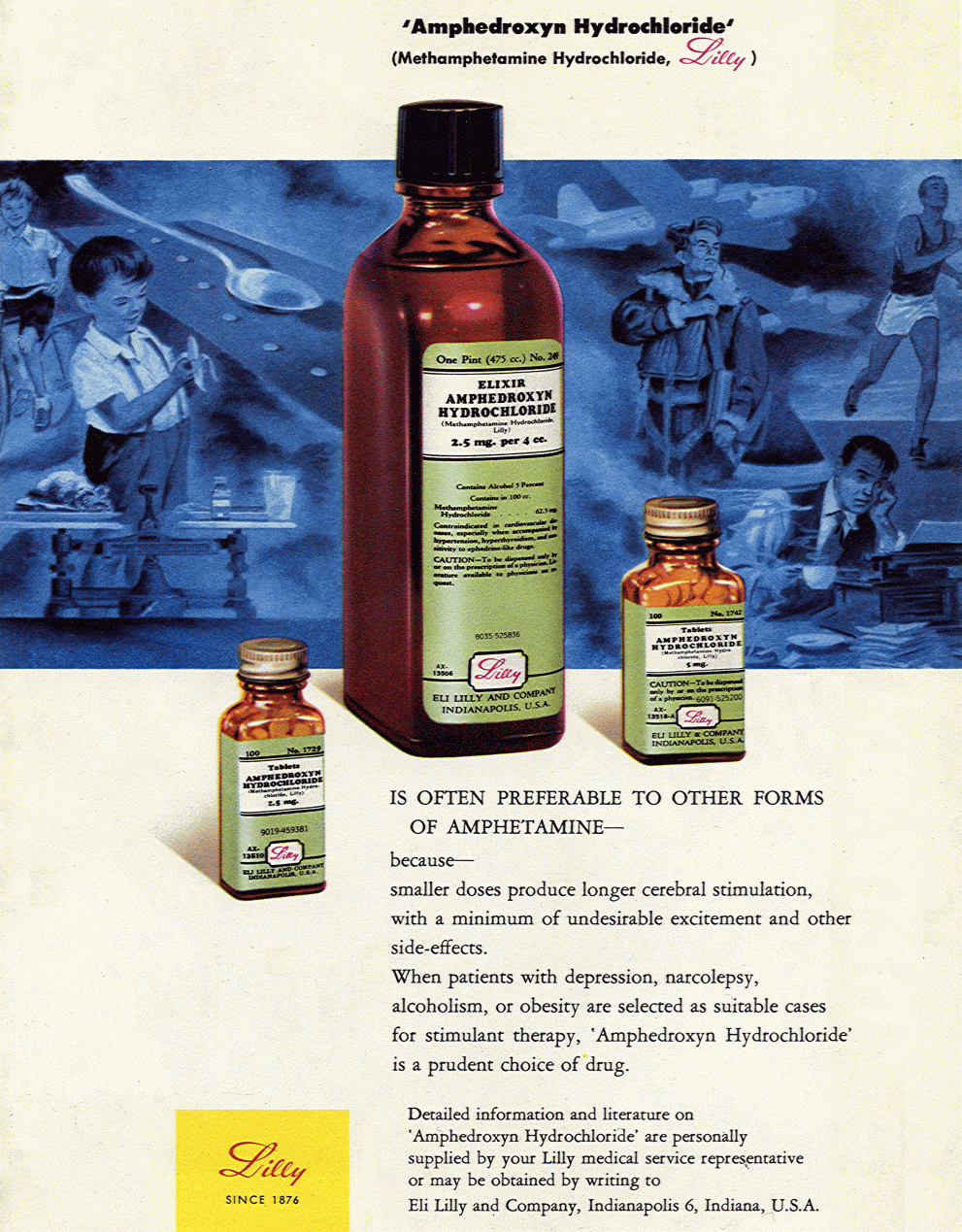
Eli Lilly Amphedroxyn (methamphetamine) advertisement, 1951. New York State Journal of Medicine, Vol. 51, No. 18, page 2214. Elixir Amphedroxyn Hydrochloride (Methamphetamine Hydrochloride, Lilly) IS OFTEN PREFERABLE TO OTHER FORMS OF AMPHETAMINE ~ because ~ smaller doses produce longer cerebral stimulation, with a minimum of undesirable excitement and other side-effects. When patients with depression, narcolepsy, alcoholism, or obesity are selected as suitable cases for stimulant therapy, Amphedroxyn Hydrochloride is a prudent choice of drug. Contraindicated in cardiovascular diseases, especially when accompanied by hypertension, hyperthyroidism, and sensitivity to ephedrine-like drugs. CAUTION ~ To be dispensed only by or on the prescription of a physician. Literature available to physicians on request. Detailed literature on Amphedroxyn Hydrochloride are personally supplied by your Lilly medical service representative or may be obtained by writing to Eli Lilly and Company, Indianapolis 6, Indiana, U.S.A. LILLY Since 1876 Methamphetamine - The only feature that greatly distinguishes this congener of amphetamine from amphetamine itself is the exceedingly large number of trade names under which this material is sold: to name some, Amphedroxyn, Apamine, Deofed, Desamine, Desoxedrine, Desoxo-5, Desoxyephedrine, Desoxyn, Desvphed, Detrex, Dexoval, Dexstim, D-O-E, Doxyfed Drinalfa, Efroxine, Lanazine, Methamphin, Methedrine, Methoxyn, Miller-Drine, Xorodin, Oxyfed, Oxydess, Premodrin, Normadrine, Xorodin, Semoxydrine, Stimdex, and Syndrox. There is no evidence that its action differs from that of amphetamine in any way except that the action on the cardiovascular system is somewhat less intense and the action on the central nervous system is somewhat more intense. This drug is probably abused more than any other of the group. -- Diet pill (amphetamines) traffic, abuse and regulation: hearings before the Subcommittee to Investigate Juvenile Delinquency of the Committee on the Judiciary, United States Senate, 92nd Congress, February 1972. |