British Medical Journal, Vol. 1, No. 5242, p. 12.
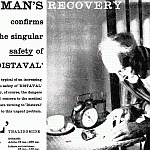
this child's life may depend on the safety of Distaval
Consider the possible outcome in a case such as this -- had the bottle contained a conventional barbiturate. Year by year, the barbiturates claim a mounting toll of childhood victims. Yet it is simple enough to prescribe a sedative and hypnotic which is both highly effective . . . and outstandingly safe... 'Distaval' (thalidomide) has been prescribed for over three years in this country, where the accidental poisonings rate is notoriously high; but there is no case on record in which even gross overdosage with 'Distaval' has had harmful results. Put your mind at rest. Depend on the safety of DISTAVAL ~ trade mark
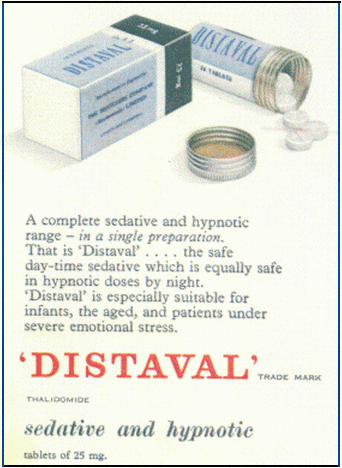
As a hypnotic at bedtime: ADULTS: 50 mg. to 200 mg. INFANTS AND CHILDREN: 25 mg. to 100 mg. As a daytime sedative: ADULTS: 25 mg. two or three times daily. INFANTS AND CHILDREN: Up to 25 mg., according to age, one to three times daily.
Distaval (25 mg. tablets). Distaval Forte (100 mg. tablets). Basic cost to N.H.S. of 12 tablets from dispensing pack of one hundred -- 1/- or 2/8d. according to strength. Distaval Suspension (50 mg. per 5 ml.) Basic cost to N.H.S. 3/- per bottle of 60 ml.
REFERENCES: Practitioner, 1959, 183, 57. J. clin. exp. Psychopath., 1959, 20, 243. J. Coll. gen. Pract., 1958, 1, 398. Brit. med. J., 1959, 2, 635. Med. Wld. (Lond.), 1960, 93, 26. Brit. J. Pharmacol., 1960, 15, 111.
DCBL ~ The Distillers Company (Biochemicals) Limited
Broadway House, The Broadway, Wimbledon, London, S.W.19 Telephone: LIBerty 6600 Owners of the trade mark 'Distaval'